News and Information
Message from First Responders regarding COVID-19

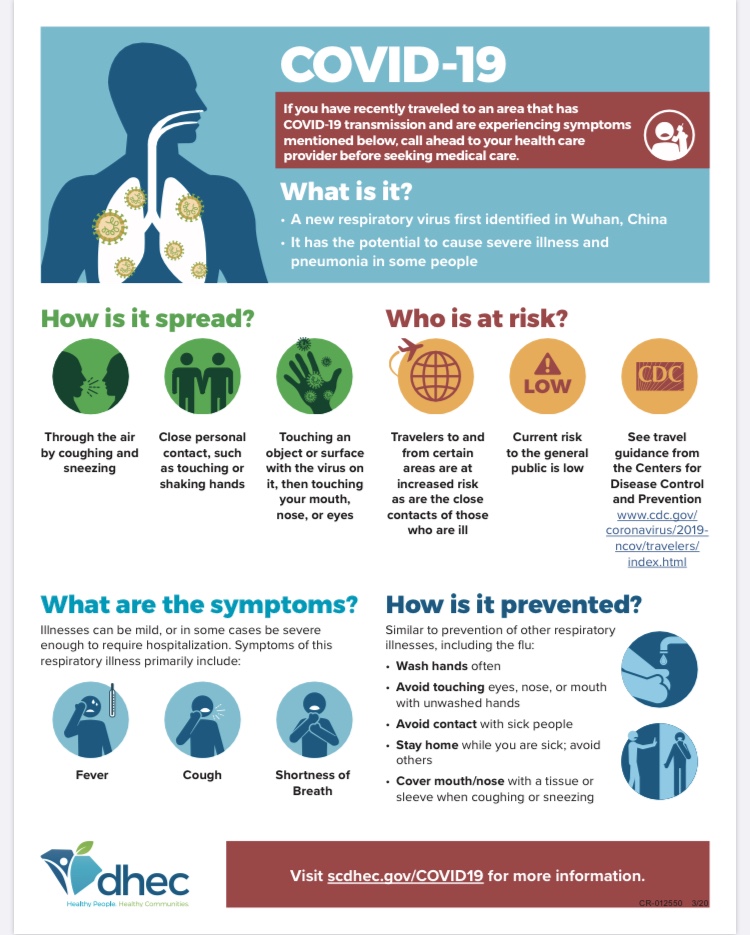
Corona Virus Information (COVID-19)



North Carolina 2019 Paramedic Competition
Beaufort EMS Director Donna Ownby and retired Beaufort EMS Director Rusty Hollingsworth were again invited to judge the annual North Carolina (NC) Paramedic Competition at the NC EMS Expo. Hosted by the Office of Emergency Medical Services, NC College of Emergency Physicians, and NC Association of EMS Curriculum Educators, the 29th Annual Paramedic Competition was held September 29, 2019 in Greensboro, NC. Winning teams from 5 regional preliminary sites, along with the 2018 defending champions faced a challenging scenario made incredibly realistic using both live role players and high-fidelity simulation mannequins staged on a realistic beach set. This year’s scenario began with a 20-year-old shark attack victim with a left lower leg amputation and a significant bite to the torso. As soon as EMS arrives on scene, a woman enters carrying an unresponsive 9-year-old (simulation mannequin) who was frightened by the shark attack, panicked, disappeared under water and has no breathing or pulse. At the 6-minute mark, a woman walking on the beach appears stating that her sister, 7 months pregnant, is in labor. The simulation mannequin delivers a pre-term infant (also a mannequin). The teams were evaluated on their management of the four patients.
Congratulations to paramedics John Stroup III and Michael Dudkowski of Mecklenburg Emergency Medical Services Agency, who won this year’s competition.


Beaufort County EMS Receives Pet Oxygen Mask Donation
Beaufort County EMS recently received a donation of pet oxygen masks from Invisible Fence. Every ambulance in Beaufort County will now have a kit available to help any animals that might need help during an emergency. When our Beaufort County EMS and other first responders answer emergency calls, they will now be able to more effectively help our four-footed family members. Thank you to Beaufort County EMS, Beaufort County Animal Services and Janice Alden from Invisible Fence. Big thank you to our team at The County Channel for putting together a terrific video. Watch the video here: https://youtu.be/V1YcZo5GyPk
Burton Fire District to honor boat captain who saved man’s life
By Kristen Rary September 20, 2019 at 4:35 PM EDT - Updated September 20 at 4:53 PM
BURTON, S.C. (WTOC) - The Burton Fire District will honor a charter boat captain Friday night in a ceremony that is meant to reflect the department’s admiration for community members that step up and become heroes.
Benjamin Ranney was taking a couple out on his chartered fishing boat when he noticed an emergency happening across the water.
Officials are still fuzzy on the details, but what they do know is that Ranney was able to get to the scene, position his boat next to a man that was bobbing in the water about to go under, and lift him out before he could.
After the man got to safety, Ranney went back about his day, but the wife of the man who was saved insisted they find the rescuer. She reached out to the Burton fire District for help. Together, they not only identified Ranney, but they have decided to honor him with an award.
Burton Fire Captain Dan Byrnes says Ranney stopped a tragedy by acting in the moment.
“If he had just called 911 and waited for us to respond, by the time we got there, we would’ve had a tragedy and a fatality, but he didn’t. He acted, which is what we want to educate and train our citizens to do, and made the most of the seconds, and he was able to get him out of the water and get him to the shore," Byrnes said. "We were able to take it from there and transport him to the hospital and get him the care he needed, and he’s doing well. He’ll be there to thank the family tonight.”
Ranney is a trained boat captain, and knew what to do in case of an emergency, but the Burton fire District still applauds him and anyone who acts bravely and prepares for any worst case scenario. It’s why they teach CPR and other emergency defenses for free to community members.
The ceremony will be held at 6 p.m. at the main Burton Fire District office. They say they are expecting just family and friends, but anyone is welcome to come and show their support for the man who saved a life.
Copyright 2019 WTOC. All rights reserved.
Outbreak of Lung Injury Associated with E-Cigarette Use, or Vaping
Posted September 27, 2019 at 1:00pm ET
CDC, the U.S. Food and Drug Administration (FDA), state and local health departments, and other clinical and public health partners are investigating a multistate outbreak of lung injury associated with e-cigarette product use, or vaping.
Key Facts about E-Cigarette Use, or Vaping
- Electronic cigarettes – or e-cigarettes — are also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems (ENDS).
- Using an e-cigarette product is commonly called vaping.
- E-cigarettes work by heating a liquid to produce an aerosol that users inhale into their lungs.
- The liquid can contain: nicotine, tetrahydrocannabinol (THC) and cannabinoid (CBD) oils, and other substances and additives. THC is the psychoactive mind-altering compound of marijuana that produces the “high”.
What we know
- There are 805* lung injury cases reported from 46 states and 1 U.S. territory. Twelve deaths have been confirmed in 10 states.
- CDC has received sex and age data on 771 patients.
- About 69% of patients are male.
- Nearly two thirds (62%) of patients are 18 to 34 years old; with 22% of patients between 18-21.
- 16% of patients are under 18 years.
- All reported patients have a history of e-cigarette product use or vaping.
- The latest findings from the investigation into lung injuries associated with e-cigarette use, or vaping, suggest products containing THC play a role in the outbreak.
- CDC has received data on substances used in e-cigarettes or vaping products in the 30 days prior to symptom onset among 514 patients.
- About 77% reported using THC-containing products; 36% reported exclusive use of THC-containing products.
- About 57% reported using nicotine-containing products; 16% reported exclusive use of nicotine-containing products.
- CDC has received data on substances used in e-cigarettes or vaping products in the 30 days prior to symptom onset among 514 patients.
What we don’t know
- The specific chemical exposure(s) causing lung injuries associated with e-cigarette product use, or vaping, remains unknown at this time.
- No single product or substance has been linked to all lung injury cases.
- More information is needed to know whether one or more e-cigarette or vaping products, substances, or brand is responsible for the outbreak.
What CDC recommends
- While this investigation is ongoing, CDC recommends that you consider refraining from using e-cigarette, or vaping, products, particularly those containing THC.
- If you are an adult who used e-cigarettes containing nicotine to quit cigarette smoking, do not return to smoking cigarettes.
- If you have recently used an e-cigarette or vaping product and you have symptoms like those reported in this outbreak, see a healthcare provider.
- Regardless of the ongoing investigation:
- Anyone who uses an e-cigarette or vaping product should not buy these products (e.g., e-cigarette or vaping products with THC or CBD oils) off the street, and should not modify or add any substances to these products that are not intended by the manufacturer.
- Youth and young adults should not use e-cigarette, or vaping, products.
- Women who are pregnant should not use e-cigarette, or vaping, products.
- Adults who do not currently use tobacco products should not start using e-cigarette, or vaping, products.
Latest Outbreak Information on Lung Injury Associated with Electronic Cigarettes, or Vaping
- As of September 24, 2019 at 5pm, 805* lung injury cases associated with the use of e-cigarette or vaping products have been reported to CDC from the following states and 1 U.S. territory: AR, AZ, CA, CO, CT, DE, FL, GA, HI, IA, ID, IL, IN, KS, KY, LA, MA, ME, MD, MI, MN, MO, MT, MS, NC, ND, NE, NJ, NM, NV, NY, OH, OK, OR, PA, SC, SD, TN, TX, UT, VA, VT, WA, WI, WV, WY, and USVI. These numbers may change frequently.
- Twelve deaths have been confirmed in California (2), Florida, Georgia, Illinois, Indiana, Kansas (2), Minnesota, Mississippi, Missouri, and Oregon.
- The latest findings from the investigation into lung injuries associated with e-cigarette use, or vaping, suggest products containing THC play a role in the outbreak.
- Most of the patients reported using THC-containing products or both THC-containing products and nicotine-containing products. Some of the patients reported using only nicotine-containing products.
- All patients have a reported history of e-cigarette product use, or vaping, and no consistent evidence of an infectious cause has been discovered. Therefore, the suspected cause is a chemical exposure.
- The specific chemical exposure(s) causing lung injuries associated with e-cigarette product use, or vaping, remains unknown at this time.
- No single product or substance has been linked to all lung injury cases. More information is needed to know whether a single product, substance, brand, or method of use is responsible for the outbreak.
- CDC has received sex and age data on 771 patients.
- About 69% of patients are male.
- Nearly two thirds (62%) of patients are 18 to 34 years old; with 22% of patients between 18-21.
- 16% of patients are under 18 years.
- All reported patients have a history of e-cigarette product use or vaping.
- The latest findings from the investigation into lung injuries associated with e-cigarette use, or vaping, suggest products containing THC play a role in the outbreak.
- CDC has received data on substances used in e-cigarettes or vaping products in the 30 days prior to symptom onset among 514 patients.
- About 77% reported using THC-containing products; 36% reported exclusive use of THC-containing products.
- About 57% reported using nicotine-containing products; 16% reported exclusive use of nicotine-containing products.
- CDC has received data on substances used in e-cigarettes or vaping products in the 30 days prior to symptom onset among 514 patients.
- CDC worked with states to create a case definition to classify confirmed and probable cases in a consistent way. State investigators determine if cases are confirmed or probable after examining the medical records of suspected patients and consulting with the clinical care team to exclude other possible causes of illness. Unlike nationally reportable conditions, these cases are requiring clinicians and public health professionals to interview patients to determine product use and individual behaviors.
- CDC will report numbers of confirmed and probable lung injury cases once states have finalized their classification of cases.
- States are in the process of classifying patients. We expect that states and clinicians may look back for past lung injury cases based on CDC’s case definition.
- This complex investigation spans many states, involves hundreds of patients, and involves a wide variety of substances and e-cigarette, or vaping, products.
- CDC continues to work closely with FDA, states, public health partners, and clinicians on this outbreak.
*The increase in lung injury cases from last week represents both new patients and recent reporting of previously-identified patients to CDC.
Click on the following link for the original full article - Outbreak of Lung Injury Associated with E-Cigarette Use, or Vaping
Flu Symptoms Everyone Should Be Aware of as Flu Season Approaches
By Renee Cherry September 19, 2019
Wondering if you've got the flu or something else? Here's your guide to influenza symptoms you should watch out for.
The phrase "flu-like symptoms" is used to describe the effects of everything from the initial stages of an STD and jet lag to a result of adopting a keto diet. But what are these symptoms that are getting compared to the flu (a really serious illness btw), exactly? If you don't know from firsthand experience—consider yourself lucky—read on for a full rundown for the sign of influenza everyone should know how to spot. (Related: When Is the Best Time to Get a Flu Shot?)
Signs and Symptoms of the Flu or Influenza
In short, influenza is no walk in the park. Fever, chills, cough, muscle or body aches are all common flu symptoms, and some people also experience diarrhea or vomiting, says flu expert Norman Moore, Ph.D., director of infectious diseases scientific affairs for Abbott. "These can come on suddenly and often point to influenza, but someone with the flu might not necessarily experience all of these symptoms," says Moore. "For instance, not everyone with the flu spikes a fever, and children are more likely than adults to have vomiting and diarrhea." (Side note: While some people have gastrointestinal symptoms with the flu, gastroenteritis a.k.a the stomach flu is a separate virus and illness that doesn't affect your respiratory system.)
How Dangerous Is the Flu?
Influenza symptoms can be severe and even deadly. During the 2017-2018 flu season in the US, more than 959,000 people were hospitalized and over 79,400 people died, according to the CDC's estimates. And the aforementioned flu symptoms aren't the only thing you have to worry about. "Flu symptoms such as fever, diarrhea, vomiting, and fatigue can be severe, but the most dangerous consequences of the flu are its complications," says Moore. "For example, flu virus infection may lead to sinus and ear infections, pneumonia, organ failure, sepsis, and even death. Additionally, flu also can worsen chronic medical problems such as asthma and heart disease." Children, people aged 65 or older, and people with chronic conditions are more at risk for serious symptoms, according to the CDC. (Related: Can You Get the Flu Twice In One Season?)
How Is the Flu Diagnosed and Treated?
Doctors often diagnose the flu based on symptoms alone, but sometimes they'll suggest you take a flu test for added certainty that you're dealing with the flu and not another health issue. The swab tests are administered at doctors' offices, clinics, urgent care centers, and ERs. Making the effort to seek a diagnosis if you're experiencing flu symptoms is beneficial, says Moore. "Getting the right diagnosis as soon as possible is important because it allows people with the flu to start treatment earlier when those treatments work best. Without testing, patients are more likely to receive antibiotics inappropriately—for example, if a patient actually has the flu or another viral illness," he says. "Not only does the inappropriate use of antibiotics not help someone with the flu, it can lead to more antibiotic-resistant bacteria that could do further harm." (Related: How Quickly Can You Really Catch an Illness On an Airplane—and How Much Should You Worry?)
The flu doesn't always look the same; It can vary from person to person, with any combo of symptoms and severity levels. But one thing you can always count on is that if you're dealt with the flu, it'll be a miserable ordeal.
Click on the following link for full article - Flu Symptoms
FDA approves first of its kind device to treat pediatric patients with progressive idiopathic scoliosis
Published August 16, 2019
The U.S. Food and Drug Administration today approved the first spinal tether device intended to be used in children and adolescents to correct the most common form of scoliosis, called idiopathic scoliosis, that has not responded to conservative treatment options, such as external bracing. The device, called The Tether – Vertebral Body Tethering System, is intended to treat growing children and adolescents whose spinal curves are approaching or have reached the range where surgical treatment is an option.
“For children and adolescent patients with idiopathic scoliosis that does not respond favorably to bracing, treatment options have been limited to fusion surgeries,” said Capt. Raquel Peat, Ph.D., director of the Office of Orthopedic Devices in the FDA’s Center for Devices and Radiological Health. “Today’s approval provides access to a new treatment option that could improve quality of life for patients with idiopathic scoliosis.”
Idiopathic scoliosis is a sideways curvature of the spine whose cause is unknown. It is the most common spinal deformity in children and is most often diagnosed between ages 10 to 18, although it may occur at a younger age. The standard treatments for idiopathic scoliosis among children and adolescents who are still growing are conservative, non-surgical treatments such as external bracing to help correct the spinal curvature. Approximately 6,800 patients in the U.S. each year will develop progressive curvatures that do not respond to bracing. For these patients, spinal fusion surgery (i.e., spinal implants to correct the curvature of the spine and fusion surgery) may be used to permanently stabilize and correct spinal curvatures. While spinal fusion is often successful, this surgery permanently restricts the motion of the spine and may have long-term complications such as pain, arthritis and future spinal deformities, which could require additional surgical treatment.
The Tether – Vertebral Body Tethering System provides an alternative for patients with idiopathic scoliosis that doesn’t respond to bracing. As a patient grows, The Tether – Vertebral Body Tethering System is designed to continue to correct the curvature while maintaining a fuller range of motion when compared to spinal fusion procedures.
The Tether – Vertebral Body Tethering System includes anchors and vertebral body screws that are placed into the same side of each vertebra in the curved section of the spine through an incision on the side of the chest. A flexible cord, called a tether, is connected to the screws. Tension is applied to the tether during surgery to compress one side of the spine and to partially correct the curve. Over time, the tether slows growth on the curved side of the spine and promotes growth on the opposite side. This provides additional correction of the curve as the patient continues to grow. The Tether - Vertebral Body Tethering System is not intended to be removed unless certain problems, such as overcorrections, develop. Health care professionals will monitor patients, conducting follow-up x-rays, to track the spinal curvature and identify any potential problems that might require additional surgery to revise or remove the device. For those patients whose curves are not adequately corrected by The Tether – Vertebral Body Tethering System, spinal fusion surgery is still possible.
The FDA reviewed data for The Tether – Vertebral Body Tethering System through the humanitarian device exemption (HDE) process. A Humanitarian Use Device (HUD) is a device intended to benefit to patients by treating or diagnosing a disease or condition that affects not more than 8,000 individuals in the U.S. per year.
The FDA reviewed clinical data supporting the safety and probable benefit of The Tether – Vertebral Body Tethering System from 57 patients who received the device. At two years, 43 patients had sufficient improvement of the curvature of their spines and did not need spinal fusion. The most common serious adverse events observed included overcorrection of the curvature, tether breakage, and pneumothorax or air leakage into the space between the lung and chest wall. General complications consistent with any spinal surgical procedure were also noted including pain, respiratory problems, nerve injuries and bleeding.
Zimmer Biomet Spine has shared with the FDA that it will be partnering with the Harms Study Group, a cohort of surgeons dedicated to the advancement of treatment for children and adolescents with spinal deformities, to develop a patient data registry to help assess the long-term performance of The Tether System.
“The FDA continues to collaborate with stakeholders to encourage the development of registries, including the one being developed for this device, as an additional tool to gather and track real world evidence,” said FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D. “This type of post-market data generation can provide ongoing device safety surveillance and additional evidence for effectiveness. More broadly, real world evidence can help support innovative developments while ensuring that patient health and safety remains the top priority.”
The FDA granted the approval of The Tether - Vertebral Body Tethering System to Zimmer Biomet Spine.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
[To see this article on the FDA website, please click on the following link - FDA approves first of its kind device to treat pediatric patients with progressive idiopathic scoliosis]
FDA approves pembrolizumab for advanced esophageal squamous cell cancer
On July 30, 2019, the Food and Drug Administration approved pembrolizumab (KEYTRUDA, Merck) for patients with recurrent, locally advanced or metastatic, squamous cell carcinoma of the esophagus (ESCC) whose tumors express PD-L1 (Combined Positive Score [CPS] ≥10), as determined by an FDA-approved test, with disease progression after one or more prior lines of systemic therapy.
FDA also approved a new use for the PD-L1 IHC 22C3 pharmDx kit as a companion diagnostic device for selecting patients for the above indication.
Efficacy was investigated in two clinical trials, KEYNOTE‑181 (NCT02564263) and KEYNOTE‑180 (NCT02559687). KEYNOTE-181 was a randomized, open-label, active-controlled trial that enrolled 628 patients with recurrent locally advanced or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced or metastatic disease. Patients were randomized (1:1) to receive either KEYTRUDA 200 mg intravenously (IV) every 3 weeks or the investigator’s choice of the following regimens: paclitaxel 80-100 mg/m2 IV on days 1, 8, and 15 of every 4‑week cycle; docetaxel 75 mg/m2 IV every 3 weeks; or irinotecan 180 mg/m2 IV every 2 weeks (control arm). Randomization was stratified by geographic region and histologic subtype (squamous versus adenocarcinoma). PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx kit.
The primary efficacy outcome measure of KEYNOTE-181 was overall survival (OS) in patients with ESCC, patients with tumors expressing PD-L1 CPS ≥10, and all randomized patients. Additional efficacy outcome measures were progression-free survival (PFS), overall response rate (ORR), and response duration. The hazard ratio for OS in patients with ESCC whose tumors expressed PD-L1 CPS ≥10 was 0.64 (95% CI: 0.46, 0.90). Median OS was 10.3 months (95% CI: 7.0, 13.5) and 6.7 months (95% CI: 4.8, 8.6) in the pembrolizumab and control arms, respectively.
KEYNOTE‑180 was a single arm, open-label trial that enrolled 121 patients with locally advanced or metastatic esophageal cancer who progressed on or after at least 2 prior systemic treatments for advanced disease. With the exception of the number of prior lines of treatment, the eligibility criteria were similar to and the dosage regimen identical to KEYNOTE-181.
The major efficacy outcome measures of KEYNOTE-180 were ORR and response duration. In the 35 patients with ESCC expressing PD-L1 CPS ≥10, ORR was 20% (95% CI: 8, 37) and response durations ranged from 4.2 to 25.1+ months, with 71% (5 patients) having responses of 6 months or longer and 57% (3 patients) having responses of 12 months or longer.
Adverse reactions in patients with esophageal cancer were similar to those in 2,799 patients with melanoma or NSCLC treated with single-agent pembrolizumab. Common adverse reactions reported in at least 20% of patients receiving pembrolizumab include fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain.
The recommended pembrolizumab dose for esophageal cancer is 200 mg every 3 weeks.
View full prescribing information for KEYTRUDA.
FDA granted these applications priority review. A description of FDA expedited programs is in the Guidance for Industry: Expedited Programs for Serious Conditions-Drugs and Biologics.
Healthcare professionals should report all serious adverse events suspected to be associated with the use of any medicine and device to FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
Check out recent approvals at the OCE’s podcast, Drug Information Soundcast in Clinical Oncology (D.I.S.C.O.).
For assistance with single-patient INDs for investigational oncology products, healthcare professionals may contact OCE’s Project Facilitate at 240-402-0004 or email OncProjectFacilitate@fda.hhs.gov.
Follow the Oncology Center of Excellence on Twitter @FDAOncology.
[To see this article on the FDA website, please click on the following link - FDA approves pembrolizumab for advanced esophageal squamous cell cancer.]
South Carolina Hurricane Guide
Available online at https://scemd.org/stay-informed/publications/hurricane-guide.
The South Carolina Hurricane Guide is updated annually by SCEMD in collaboration with local and state partners of the S.C. Emergency Response Team. All information contained in the Guide is valid at the time of publishing each year, but is subject to change depending on actual storm conditions.
To request bulk orders of the Hurricane Guide, please send your desired quantity and street address to pio@emd.sc.gov.
South Carolina Operational Conditions
The South Carolina Emergency Management Division operates on a system of Operational Condition Levels, also known as OPCONS. This numerical scale is how SCEMD, the State Emergency Response Team and counties coordinate, prepare and respond to major emergencies.
The levels are designed to simplify the steps agencies take in order to fully activate emergency resources. The three OPCONs and their definitions are compatible with the majority of state and federal emergency management organizations nationwide, making the state’s processes and procedures easier to understand for teams deploying into South Carolina during a disaster.
South Carolina’s OPCONs and their definitions are as follows:
OPCON ONE - Full Alert [red color per SCEMD web page]
A disaster or emergency is imminent or occurring. The State Emergency Operations Center is fully activated. All agency personnel are activated or available for activation.
OPCON TWO - Enhanced Awareness [yellow color per SCEMD web page]
A disaster or emergency is likely to affect the state. Emergency Operations Plans are implemented. The State Emergency Operations Center is partially activated if necessary.
OPCON THREE - Normal Daily Operations [blue color per SCEMD web page]
Agencies coordinate, plan, train and exercise as warranted. Incidents are monitored by the State Warning Point and local emergency managers.
Click on the following link to see official story at SCEMD - Operational Condition Levels Opcon
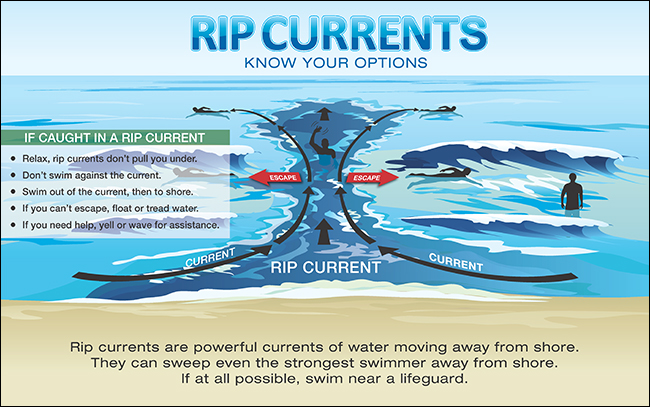
Break the Grip of the Rip Tide
Beaufort County Emergency Management -
Do you know how to spot a rip current? If you get caught in one, do you know how to get out of it?
A typical rip current ranges from 50-100 feet wide, and can extend 100 yards or more offshore. It can reach speeds of over 5 miles per hour - that’s faster than an Olympic swimmer - and can be deadly.
For more information on rip currents, visit https://www.weather.gov/safety/ripcurrent-media
Coastline: Health & Safety [in the Heat for humans and pets, CPR, AED]
A new episode of The County Channel’s series Coastline discusses health & safety. Joining host Rick Forschner is Pam Toney, executive director for the Greater Bluffton & Jasper Volunteers in Medicine. We also talk about your safety in the heat, with EMS crew chief AJ Drake & EMS Training Coordinator Karen Morris. In high heat temperatures of the day, drink plenty of fluids to stay hydrated. Find places with air conditioning. Libraries, shopping malls, and community centers can provide a cool place to take a break from the heat.
Click on following link for full video - Coast Line. Beaufort County EMS interview begins at around minute 12.
Smart911 Safety Profiles
June 14 at 10:27 AM
Protect you and your loved ones by creating a Smart911 Safety Profile. You can provide 9-1-1 with life-saving information in an emergency and receive important community and weather alerts. Download the Smart911 app to your smartphone or go to www.smart911.com to create your profile today.
Facebook - Beaufort County Emergency Management - Smart 911

Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium/Hydrochlorothiazide Tablets, USP
Company Announcement - April 22, 2019
Torrent Pharmaceuticals Limited is expanding its recall for Losartan Potassium Tablets USP and Losartan Potassium/hydrochlorothiazide tablets, USP, to the consumer level due to the detection of trace amounts of an unexpected impurity found in an active pharmaceutical ingredient (API) manufactured by Hetero Labs Limited.
The Recall is expanded to include an additional 36 lots of Losartan potassium Tablets USP and 68 lots of Losartan Potassium/Hydrochlorothiazide Tablets, USP
The impurity detected in the API is N-Methylnitrosobutyric acid (NMBA). Torrent is only recalling lots of losartan-containing products that contain N-Methylnitrosobutyric acid (NMBA) above the acceptable daily intake levels released by the FDA.
To date, Torrent Pharmaceuticals Limited has not received any reports of adverse events related to this recall.
Losartan is used to treat hypertension, hypertensive patients with Left Ventricular Hypertrophy and for the treatment of nephropathy in Type 2 diabetic patients. Losartan potassium and hydrochlorothiazide tablets, USP is used to treat hypertension and hypertensive patients with Left Ventricular Hypertrophy.
Patients who are taking Losartan Potassium Tablets, USP and Losartan Potassium/Hydrochlorothiazide Tablets, USP should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication.
The product/lots included in the expanded recall are listed below in red. The product can be identified by checking the product name, manufacturer details and batch or lot number on the bottle containing these products.
Click on the link for more details about the voluntary recall: Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium/Hydrochlorothiazide Tablets, USP
Beaufort County EMS Paramedic named South Carolina Paramedic of the Year

By Lyndsey Gough | April 11, 2019 at 9:55 PM EDT - Updated April 11 at 11:36 PM
BLUFFTON, SC. (WTOC) - If you have to call for an ambulance in Beaufort County, take comfort knowing that you’re in good hands. The Lowcountry is home to an award-winning paramedic.
Fire Station number 30 in Bluffton is home to South Carolina’s 2019 Paramedic of the Year.
“It was the first time they saw me speechless,” Angie Stewart said with a laugh.
Stewart said she was surprised, but honored, to be named SC’s Paramedic of the Year at the Annual South Carolina EMS Symposium in Myrtle Beach.
This award goes to the paramedic who has contributed most significantly to EMS at the community, state or national level.
“She’s really great with her medicine," said fellow paramedic, Mitchell Chapman. "She has even better bedside manner. I’ve seen some people in the field where they get burned out and they become a little cold, but she has a certain kind of hospitality that she bestows upon her patients and it allows them to be able to trust her.”
Chapman is Stewart’s partner in the ambulance.
“It’s very humbling, because the group of people here, even in Beaufort, I work around some fantastic paramedics. The group of people that I work with in FAST are fantastic," Stewart added.
FAST stands for First Responders Assistance and Support Team - a group Stewart is very active with. Her work with FAST, in addition to her paramedic work, earned her the nomination for the award. With this added spotlight, she’s hoping to bring more awareness to the team. Right now, they are made up of around 100 members from across the state.
'It’s a volunteer group made up for people from across the state and what we do is offer services, peer groups and stuff like that to people who go through difficult times. We have one of the highest suicide rates. I’m twice as likely to die from my own hands as I am the hazards of the job," Stewart explained.
“I come from 26 years ago. The culture basically was ‘you know what you got in to, suck it up.’ We put the Superman cape on us. We think we’re super human. We’re changing that culture and so it’s making it easier for people to say, 'you know what? I need to go talk to somebody," she explained.
Stewart says the most rewarding part of her job is simply people saying “thank you” after she has been able to help them.
She has worked in Beaufort County for the last four years. Additionally, she volunteers with Colleton County Fire Rescue.
“We are fortunate to have Angie here. She has been a great asset to our community and our service," Donna Ownby, Beaufort County’s EMS Director said via a press release.
Copyright 2019 WTOC. All rights reserved.
Please click on the following link for video regarding this story - Beaufort County EMS Paramedic named South Carolina Paramedic of the Year
WSAV - Jacob Kits Now in All Burton Fire District Schools
February 25, 2019
Teachers around Beaufort County have been trained to use Jacob Kits (WSAV - Jacob Kits Now In All Burton Fire District Schools) in case of a school shooting or emergency.
Tuesday marked a milestone for those kits and the local firefighter who created them.
Thanks to donations, the bags containing tourniquets and other medical items are now in every Elementary and Middle school in the Burton Fire District. All the teachers in those schools have also been trained to use them.
Burton firefighters and Beaufort County EMS placed the kits in classrooms at Whale Branch Middle School. The last school in the district to get them.


Captain Daniel Byrne and his wife created the kits in honor of Jacob Hall, an elementary school student killed in a school shooting. they believe these kits could be crucial in saving a child's life.
"Jacob Hall wanted to be a superhero and during his funeral, everyone wore superhero costumes and he told his mom he wanted to save lives and his mom said baby one day everyone will know your name," said Captain Daniel Byrne. "The fact that its grown under Jacob's name and its spread beyond Beaufort County into other counties throughout the state and throughout the nation. they know Jacob Hall's name and hopefully, they are there to save a life."
Bluffton, Lady's Island, Beaufort, and Sheldon Fire departments have also bought, installed and trained teachers on how to use the kits.
The Beaufort County Council recently voted to put one Jacob kit in all county-owned buildings.
Recall: Infant pain reliever sold at CVS and Walmart could result in vomiting, bleeding
WJCL - Updated: 11:12 AM EST Jan 31, 2019
A company says more of its products for infant ibuprofen, which could result in health problems, should be recalled.
The oral pain-reliever drops are connected with CVS Pharmacy, Walmart and Family Dollar stores and could lead to adverse health effects, such as vomiting and gastrointestinal bleeding.
The issue relates to the product potentially having higher concentrations of ibuprofen than it should.
The recalled products now apply to certain 1/2 ounce and 1 ounce bottles with February, April, August and December expiration dates for this year.
The voluntary recall comes from Tris Pharma, Inc., which manufactures the drugs that are eventually sold by retailers. According to a notice, no serious adverse events have been reported.
Please click on the following link to go to video about the - 2018 Bluffton Night Out
Beaufort County EMS Returns from Horry County
September 25, 2018 at 4:02 PM EDT - Updated September 25 at 5:18 PM
HORRY COUNTY, SC (WTOC) - The Beaufort County EMS is back home after helping with the aftermath of Hurricane Florence in Horry County.
The men headed up to South Carolina to help relieve those impacted by the storm.
“By us going up there, what we did was we allowed their ambulances to go forward and actually deal with the people that were entrapped or cut off from the regular area,” said Mark Fitzgibbons, EMT, Beaufort County EMS. “We were in a normal fire station, and we supported their normal community.”
Beaufort County EMS also sent two crews to the Myrtle Beach area to help with relief.
Please click on following link to watch video - Beaufort County EMS Returns from Horry County
Volunteer Opportunities for Hurricane Florence
BEAUFORT COUNTY EMERGENCY MANAGEMENT DIVISION
If you would like to volunteer to help with Hurricane Florence response and recovery efforts, visit Volunteer SC to register. Individuals and groups should not enter impacted areas on their own.
Learn More: Make sure your contributions get to where they need to go most by reviewing all the ways to volunteer and donate on the S.C. Emergency Management Division’s website here: https://www.scemd.org/recover/volunteer-and-donate/
Providing Aide for Horry County
At 1400 Monday, September 17, 2018, Beaufort County EMS deployed an ALS unit and crew to join the rescue and recovery efforts from hurricane Florence in the Horry County/Myrtle Beach area. Be safe Mark Fitzgibbons and Jeff Kneiling.


Medical Marijuana in Your Community presented September 5th, 2018 by the Lowcountry Alliance for Healthy Youth
Please join us at this educational and informational forum discussing medical marijuana and how it may impact your community.
Guest speakers include; Dale D. Quigley, Deputy Coordinator of HIDTA National Marijuana Initiative who will speak about the emerging effects that recreational and medical marijuana have had on communities in other states, and Major Frank O’Neal of SC Law Enforcement Division, Vice Services, who will address medical marijuana and how it may impact communities in SC.
Our 14th Judicial Circuit Solicitor, Duffie Stone, will be present to make introductions.
Please contact Wendy Cummings, RN, BSN, LCAHY Chair, with any questions concerning this event at LCAlliance4HealthyYouth@gmail.com

School Bus Safety Information
INSIDE LOOK: Saving a heart attack patient at Beaufort Memorial Hospital
BEAUFORT, S.C. (WSAV) - Beaufort Memorial Hospital sees about seventy severe heart attacks a year. Some are so serious, they have to be medevaced to the Medical University of South Carolina.
But practices like the STEMI drill keep the team's skills and speed ready to save lives.
"A heart attack in general is a lack of blood flow to a portion of the heart," said Dr. Stuart Smalheiser, a cardiologist at Beaufort Memorial (BMH).
The most dangerous is a STEMI.
"ST-Elevation Myocardial Infarction, or a STEMI, is when one artery is completely closed," Smalheiser explained. "The clock is running and literally we have to get that artery open as fast as possible."
Time is everything. Doctors have 90 minutes to save a STEMI patient.
"Get them to the Cath lab, get them on the table, get into their heart, and then once we take pictures of their heart arteries, then use wires, and balloons, and stents to open up the blockage. So we do that all within 90 minutes," he said.
A call to the Medical University of South Carolina (MUSC) means the patient needs much more serious surgery.
"They will have to go to MUSC if there's any type of like the coronary artery bypass graph, any type of Cardiothoracic surgery," said Tiffany Schweitzer, the STEMI coordinator at BMH.
BMH practices four times a year. They are one of 203 hospitals in the United States to receive the American College of Cardiology's NCDR ACTION Registry Platinum Performance Achievement Award.
"When someone is having a heart attack, time is, time is muscle, and we work diligently to save patients' lives."

New first responder stamp to be released by USPS
The stamp includes a firefighter carrying an axe, an EMS worker and a law enforcement officer shining a flashlight
Jul 20, 2018
By EMS1 Staff
MISSOULA, Mont. — The United States Postal Service will release its forever stamp honoring first responders at a dedication ceremony on Sept. 13, 2018.
The stamp, which features three first responders racing into action, was designed to recognize "all first responders for their skill, dedication and uncommon bravery," USPS wrote in a news release.
The stamp includes, from left to right, a firefighter carrying an axe, an EMS worker, with the EMS Star of Life visible on her cap, upper arm and emergency bag, and a law enforcement officer shining a flashlight.
The United States Postal Service will release the new stamp honoring first responders at a dedication ceremony on Sept. 13.
The dedication ceremony will held at the Montana Aerial Fire Depot and Smokejumper Center in Missoula, Montana.
The stamp was designed by artist Brian Stauffer, who worked with art director and designer Antonio Alcalá, and designer Ricky Altizer.









